US FDA approves ZEISS Medical’s QUATERA 700 technology
The US Food and Drug Administration (FDA) has granted approval to ZEISS Medical Technology’s QUATERA 700.
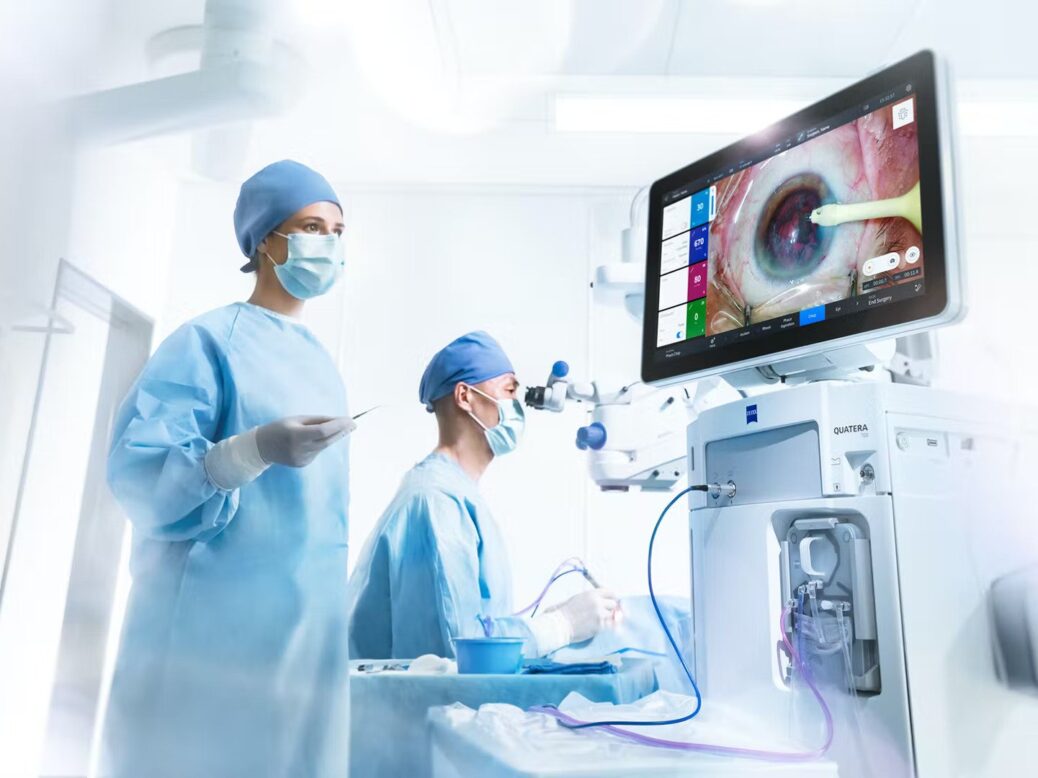
The device is intended to increase the workflow efficiency of surgeons, from the clinic to the operating room (OR).
It can be used as a single sterile OR dashboard that integrates patient data from other systems as well as the microscope view and makes it available to OR staff in real-time.
Carl Zeiss Meditec president and CEO Dr Markus Weber said: “Building on the company’s first-in-class medical technologies, ZEISS is committed to continuously expanding our innovative solutions.
“Our investment in the latest digital technologies help make customers’ workflows more efficient by integrating solutions and improving clinical results.”
QUATERA 700 includes the ZEISS QUATTRO Pump, which can deliver chamber stability independent of intraocular pressure (IOP) and flow.
The QUATTRO Pump is a synchronised fluid exchange system that can directly measure and control infusion and aspiration volumes simultaneously in real-time.
The company stated that it actively compensates for incision leakage volume.
QUATERA 700 joins all elements of the ZEISS Cataract Workflow and makes it a single sterile OR cockpit for efficient procedures.
Carl Zeiss Meditec digital business unit head and ophthalmic devices president Euan Thomson said: “ZEISS is committed to bringing our latest innovations to the United States, and the ZEISS QUATERA 700 is a great example of how integrated technology can drive efficiency in ophthalmology.
“Together with our ZEISS Medical Ecosystem and surgical planning software, we believe this fully connected and integrated approach will help set new standards for patient care and clinical management.”










Gloss