Starpharma’s DEP Delivery Technology Edge Explained In AstraZeneca’s Bcl-2/Bcl-xL Drug AZD0466 (OTCMKTS:SPHRF)
A lot of small molecule anticancer drugs have significant downsides due to poor solubility (requiring detergent additives that are toxic) and serious side effects (due to the need for high dosing to make an effective dose). Starpharma's (OCTQX:OTCQX:SPHRY) dendrimer delivery technology (DEP) is showing promise as a generic solution to these disadvantages of many small molecule cancer drugs. Until now, the DEP technology has not made an impact, although Starpharma is building an impressive portfolio in Phase II trials of resurrected old cancer drugs coupled with DEP to provide a new lease of life (and intellectual property protection). A game-changer for recognition of the DEP technology would be a big pharma company adopting the DEP technology for a new drug that has serious toxicities preventing its progression into the clinic.
AstraZeneca (NYSE:AZN) has been evaluating the Starpharma DEP technology for some time now and it recently commenced a Phase I trial on a DEP-coupled potential blockbuster drug AZD0466. Details about AZD0466 and its active ingredient AZD4320 have been unclear until the presentation of 3 posters at the recent AACR (American Association for Cancer Research) 2020 Annual Meeting. Details are provided here to assist investors in understanding this platform technology developed and owned by Australian biotech company Starpharma. DEP technology is a major value driver for Starpharma.
Astra Zeneca has 3 posters at AACR (American Association for Cancer Research) concerning AZD0466
Three posters at the AACR provide detailed information about AZD0466 (DEP-AZD4320), explaining why it is effective and how it overcomes disadvantages of the active ingredient AZD4320.
Figure 1 of the first poster “Design and optimisation of a dendrimer-conjugated dual Bcl-2/ Bcl-xL inhibitor, AZD0466, with improved therapeutic index” provides a summary of key features of AZD0466, the DEP-coupled version of active ingredient AZN4320.
These include:
1) a dendrimer composed of poly-L-lysine which has a size that keeps it in the blood (avoiding excretion in the kidneys, but small enough to accumulate in tumours)
2) a surface layer that helps solubility
3) active drug (AZC4320) exposed at the surface of the particle
4) slow release of the active drug to avoid toxicity and keep a sufficient active dose
The first poster showed a series of experiments which optimised the release half life (i.e. how quickly AZD4320 was released from the DEP dendrimer). This might sound arcane, but it made the difference between having a drug that could be taken into clinical trials and one which couldn’t.

Source: Starpharma
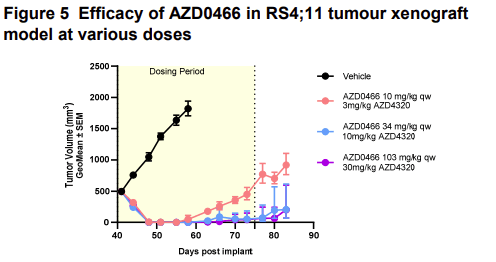
Figure 5 of the first poster shows the efficacy of AZD0466 in a tumour xenograft model. The figure shows a dose-dependent response to AZD0466, which includes increasing amounts of the active compound AZD4320 (3-30 mg/kg). At high doses, tumour growth is completely inhibited for 80 days.

Source: Starpharma
The second poster “AZD0466, a nanomedicine of a potent dual BCl-2/Bcl-xL inhibitor, exhibits anti-tumor activity in a range of haematological and solid tumor models,” talks about the mode of action of AZD0466, which is basically to get cancer cells to commit suicide. The active molecule, AZD4320, has complex activity in two different aspects of cell suicide, but it is also highly insoluble. Coupling to Starpharma’s DEP dendrimer solves the insolubility and also allows once-weekly dosing. The rest of the second poster explores a range of cancer cells that AZD0466 kills. It also shows that in combination with other anticancer agents AZD0466 becomes an effective “cure” in some cancer models.
The third poster “Anti-leukemic activity of Bcl/BclxL dual inhibitor AZD0466 in T-acute lymphoblastic leukemia preclinical models.” addressed AZD0466 activity in various preclinical leukemia models. I was not able to source this poster.
The data provided in these posters makes clear why AstraZeneca chose to partner with Starpharma to make a DEP-dendrimer version of their AZD4320 dual-action cancer drug. In a nutshell, this was because there was no path to the clinic with AZD4230, and coupling to Starpharma’s DEP technology provided a path. The posters also provide an insight into the versatility of the DEP technology to manage drug delivery, residence time in the body and solubility.
DEP technology generalises
In addition to 3 posters detailing different aspects of AZD0466, 2 posters update Starpharma’s in-house programs to rehabilitate old cancer drugs now off patent and also less used because of side effects.
A summary figure from Starpharma’s January 2020 Update indicates preclinical success of combining DEP-coupled drugs with other anti-cancer drugs, with application in pancreatic and colon cancers as well as for lymphoma.

Source: Starpharma
In a sign of confidence in its technology, Starpharma has also begun preclinical studies on DEP-coupling an unnamed major existing AstraZeneca cancer drug. The deal is that after completion of preclinical studies, AstraZeneca can licence the DEP technology for this drug for $US5 million-plus development and commercialisation milestones, plus escalating royalties on sales. If AstraZeneca doesn’t exercise the option, Starpharma has an option to license rights to develop and commercialise the DEP version of the drug in its own right or through a sub-licensee.
Risks of investing in Starpharma
Starpharma is a very small (market cap $US290 million) Australian biotech company. It has a long history of stable management and financial stability. Unlike a lot of biotech companies, there is not a history of capital raisings that dilute existing shareholders to the benefit of new investors. It has managed to attain a strong financial base that might look small but which gives it ~3 years operating cash and the prospects of being cash positive in the near term. Despite its small size, it is beginning to build a group of large conservative institutional investors who hold more than 50% of the stock.
However, the stock remains at the mercy of tired investors who keep the stock price low by selling into any price recovery. My take is that eventually these investors will all have exited and then the company will be valued on the basis of its portfolio of products that are entering international markets, and technology (primarily DEP delivery technology) which is valuable and will generalise to many significant products.
Apart from the tedium of constant share price falls between announcements, I don’t see a lot of downside with this stock and plenty of upside.
Day traders can make money off the price volatility (good liquidity on the ASX but not on the OTC listing), or long-term investors can invest at an attractive price and wait for the good times.
Conclusion
I am a long-time investor in Starpharma and I continue to be puzzled as to why this company is so neglected. Yesterday’s release of 5 posters covering new developments in the drug delivery DEP technology at the recent American Association of Cancer Research meeting helped clarify how transforming Starpharma’s DEP technology is. To date Starpharma has been repurposing previously successful cancer drugs and resolving issues that negatively impacted their use. The AstraZeneca AZD0466 story brings a new dimension by showing that a drug that has potential but serious delivery or toxicity issues can have these issues resolved through the DEP technology. This helps investors understand the value of the Starpharma technology.
The company is trading at a discount (17%) to its 12-month high, notwithstanding a series of important commercial milestones in recent months. The COVID-19 correction led to a dramatic shortfall in share price, and it is yet to recover fully from that fall. While there will be a delay until Phase I results for the AstraZeneca trial of AZN0466 become available, the results of 3 posters presented at AACR suggest that AZN0466 is likely to progress forwards. Indifference by the market is a good time to think about whether this small Australian biotech stock is worthy of a place at the high risk end of your biotech portfolio.
I am not a financial advisor, but I do understand biotech product development. If my commentary helps you and your financial advisor to evaluate a possible investment at the risky end of your biotech portfolio, please consider following me.
Disclosure: I am/we are long SPHRY. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.







Gloss