CathVision concludes patient enrolment in EP recording technology trial
CathVision has concluded patient enrolment in the clinical trial of its low-noise electrophysiology (EP) recording technology, ECGenius System, which will be the first in the US.
The University of Vermont Medical Center will initiate the technology evaluation, as the company prepares for its launch in the US.
The trial will evaluate the safety and technical performance of ECGenius and benchmark electrogram signal quality against other systems that are commercially available.
CathVision CEO Mads Matthiesen said: “ECGenius delivers a modern approach built on improved visualisation and higher quality raw data that paves the way for more informed and accurate decision-making processes in the EP lab.
“This is necessary, foundational change and will become the basis for artificial intelligence-driven therapy support and machine learning software tools.”
ECGenius has not yet received approval for commercial use in the US or Europe, but represents a key evolution in electrocardiography (ECG) signal acquisition quality, interpretation and therapy support.
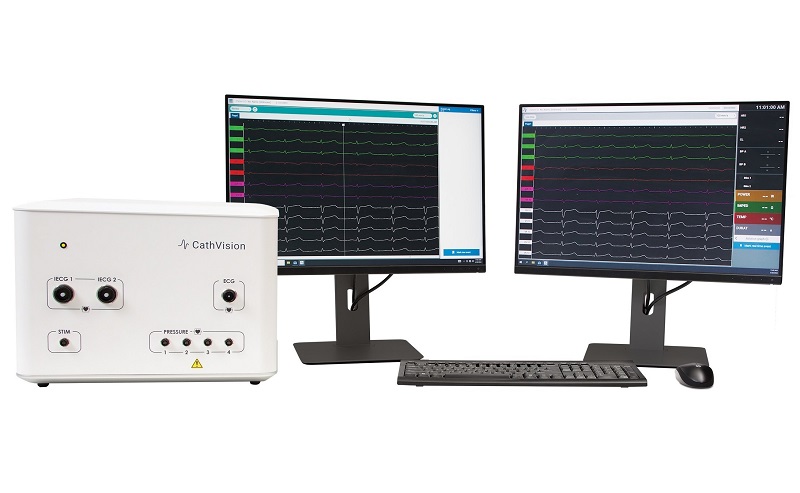
The system comprises a 12-lead ECG, four blood pressure channels and 128 intracardiac channels.
Furthermore, it can be integrated into modern hospital environments, is compatible with existing catheters and 3D mapping systems, and has been designed to guide and improve ablation therapy by receiving low-noise, high-fidelity EP signals.
The system consists of a low-noise console and computer software, offering a real-time artificial intelligence (AI) research platform to hospital partners.
Its special toolbox and algorithm catalogue enable the interpretation of intelligent electrograms.
In collaboration with additional study sites, CathVision will evaluate ECGenius and aid in the development of solutions that are based on AI and can be integrated into the system.










Gloss